Do you seek for 'how to write sulfide'? You can find all the material on this web page.
Table of contents
- How to write sulfide in 2021
- Sulphide ion
- Sulfide formula
- Sodium sulfide formula
- Sulfide symbol
- Sulphide or sulfide
- How to write sulfide 07
- How to write sulfide 08
How to write sulfide in 2021
 This image demonstrates how to write sulfide.
This image demonstrates how to write sulfide.
Sulphide ion
 This image shows Sulphide ion.
This image shows Sulphide ion.
Sulfide formula
 This picture representes Sulfide formula.
This picture representes Sulfide formula.
Sodium sulfide formula
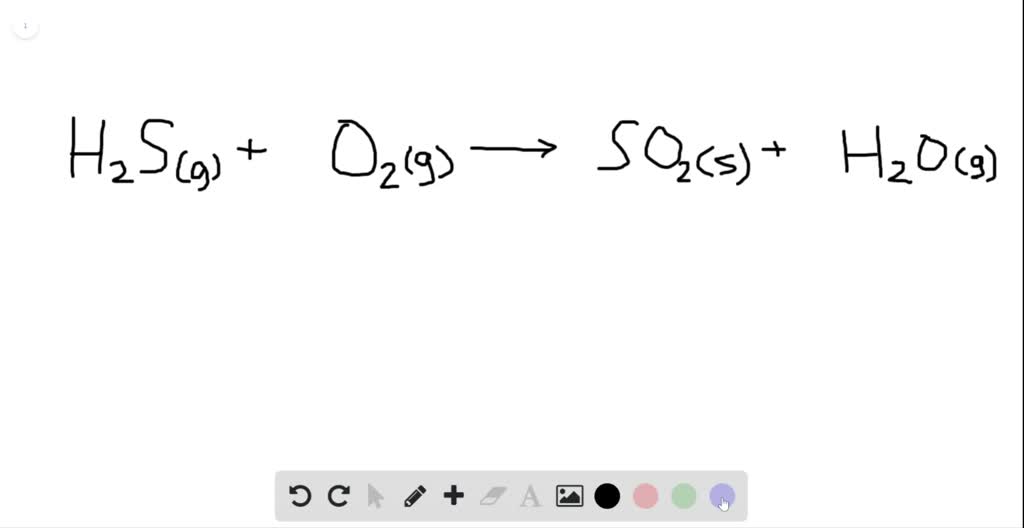 This image demonstrates Sodium sulfide formula.
This image demonstrates Sodium sulfide formula.
Sulfide symbol
 This image representes Sulfide symbol.
This image representes Sulfide symbol.
Sulphide or sulfide
 This image shows Sulphide or sulfide.
This image shows Sulphide or sulfide.
How to write sulfide 07
 This picture demonstrates How to write sulfide 07.
This picture demonstrates How to write sulfide 07.
How to write sulfide 08
 This image illustrates How to write sulfide 08.
This image illustrates How to write sulfide 08.
Which is more toxic hydrogen sulfide or organic sulfide?
Many metal sulfides are so insoluble in water that they are probably not very toxic. Some metal sulfides, when exposed to a strong mineral acid, including gastric acids, will release toxic hydrogen sulfide. Organic sulfides are highly flammable. When a sulfide burns it produces sulfur dioxide (SO 2) gas.
What is the empirical formula for Polyphenylene sulfide?
Polyphenylene sulfide (see below) has the empirical formula C 6 H 4 S. Occasionally, the term sulfide refers to molecules containing the –SH functional group. For example, methyl sulfide can mean CH 3 –SH. The preferred descriptor for such SH-containing compounds is thiol or mercaptan, i.e. methanethiol, or methyl mercaptan.
What is the empirical formula for sulfide in chemistry?
In organic chemistry, "sulfide" usually refers to the linkage C–S–C, although the term thioether is less ambiguous. For example, the thioether dimethyl sulfide is CH3–S–CH3. Polyphenylene sulfide (see below) has the empirical formula C6H4S.
What happens when hydrogen sulfide is treated with acid?
Chemical reactions. Upon treatment with a standard acid, sulfide converts to hydrogen sulfide (H2S) and a metal salt. Oxidation of sulfide gives sulfur or sulfate. Metal sulfides react with nonmetals including iodine, bromine, and chlorine forming sulfur and metal salts.
Last Update: Oct 2021