Do you hope to find 'how to write electron dot configurations'? You can find all the material on this web page.
Table of contents
- How to write electron dot configurations in 2021
- E dot diagram
- Electron dot notation
- Electron dot structure of oxygen
- Electrons dot diagram
- Electron dot configuration for oxygen
- Sodium lewis dot structure
- How to draw lewis dot structures for elements
How to write electron dot configurations in 2021
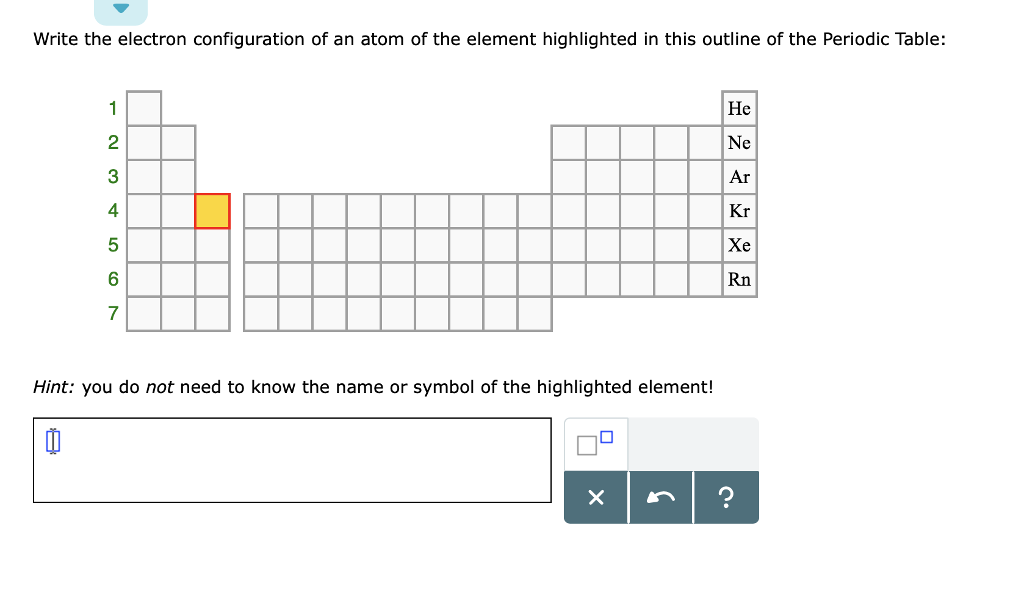 This picture shows how to write electron dot configurations.
This picture shows how to write electron dot configurations.
E dot diagram
 This picture demonstrates E dot diagram.
This picture demonstrates E dot diagram.
Electron dot notation
 This picture representes Electron dot notation.
This picture representes Electron dot notation.
Electron dot structure of oxygen
 This image shows Electron dot structure of oxygen.
This image shows Electron dot structure of oxygen.
Electrons dot diagram
 This image illustrates Electrons dot diagram.
This image illustrates Electrons dot diagram.
Electron dot configuration for oxygen
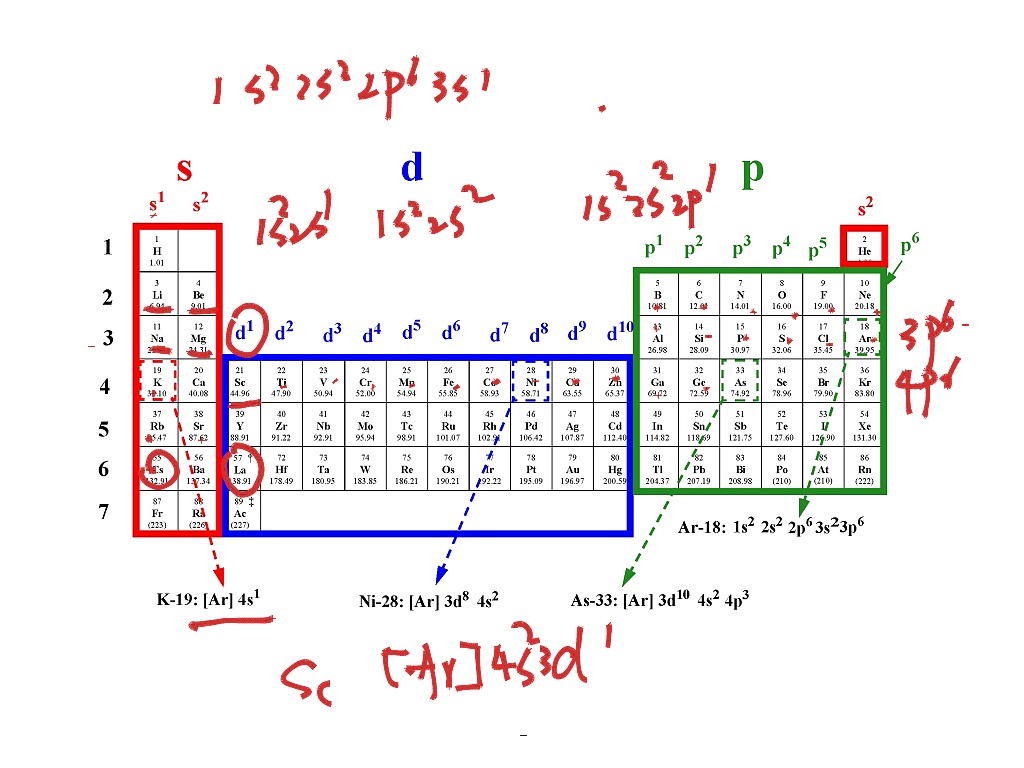 This picture representes Electron dot configuration for oxygen.
This picture representes Electron dot configuration for oxygen.
Sodium lewis dot structure
 This picture representes Sodium lewis dot structure.
This picture representes Sodium lewis dot structure.
How to draw lewis dot structures for elements
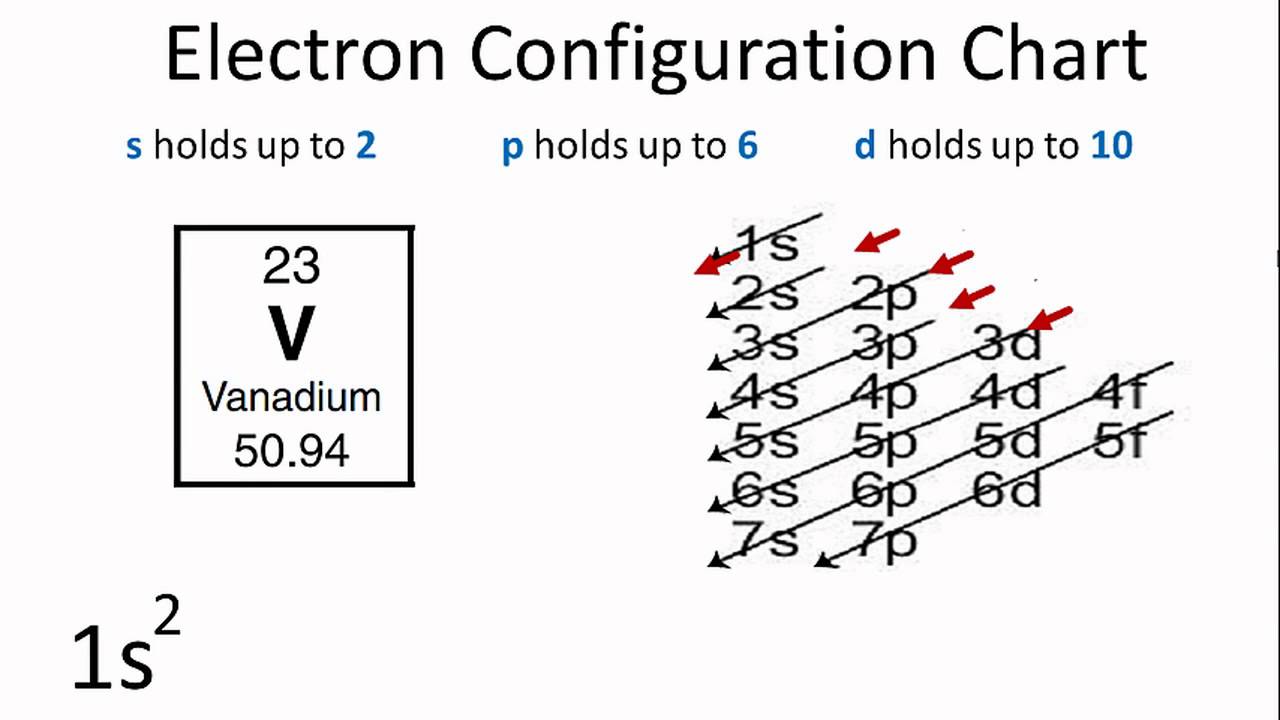 This picture representes How to draw lewis dot structures for elements.
This picture representes How to draw lewis dot structures for elements.
How do you write an abbreviated electron configuration?
Step 1 Find the symbol for the element on a periodic table. For example, to write an abbreviated electron configuration for zinc atoms, we first find Zn on the periodic table (see below). Step 2 Write the symbol in brackets for the noble gas located at the far right of the preceding horizontal row on the table.
How are quantum numbers related to electron configurations?
In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron Configuration Electron configurations are the summary of where the electrons are around a nucleus.
How to articulate the formula for an electron dot?
Stages to articulate the electron dot formula are stated beneath. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Pick up every valence electrons from every atom and toss them into a make-believe container that we can term an electron pot.
Why are there so many dots in a Lewis electron diagram?
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Last Update: Oct 2021